Raoult's law
Raoult's law states:
The vapor pressure of an ideal solution is dependent on the vapor pressure of each chemical component and the mole fraction of the component present in the solution.
Expressed as,
 |
where
- pi is the partial pressure of the component i in mixture
- p*i is the vapor pressure of the pure component i
- xi is the mole fraction of the component i in solution (in mixture)
Derivation:
We define an ideal solution as a solution for which the chemical potential of component i is: ,
,
If the system is at equilibrium, then the chemical potential of the component i must be the same in the liquid solution and in the vapor above it. That is,
 indicates reference state.
indicates reference state.The corresponding equation for pure i in equilibrium with its (pure) vapor is:
Subtracting both equations gives us
Ideal mixing
An ideal solution can be said to follow Raoult's Law but it must be kept in mind that in the strict sense ideal solutions do not exist. The fact that the vapor is taken to be ideal is the least of our worries. Interactions between gas molecules are typically quite small especially if the vapor pressures are low. The interactions in a liquid however are very strong. For a solution to be ideal we must assume that it does not matter whether a molecule A has another A as neighbor or a B molecule. This is only approximately true if the two species are almost identical chemically. We can see that from considering the Gibbs free energy change of mixing:It can be shown using the Gibbs–Duhem equation that if Raoult's law holds over the entire concentration range x = 0–1 in a binary solution then, for the second component, the same must also hold.
If the deviations from ideality are not too strong, Raoult's law will still be valid in a narrow concentration range when approaching x = 1 for the majority phase (the solvent). The solute will also show a linear limiting law but with a different coefficient. This law is known as Henry's law.
The presence of these limited linear regimes has been experimentally verified in a great number of cases.
In a perfectly ideal system, where ideal liquid and ideal vapor are assumed, a very useful equation emerges if Raoult's law is combined with Dalton's Law.
Non-ideal mixing
Raoult's Law may be adapted to non-ideal solutions by incorporating two factors that will account for the interactions between molecules of different substances. The first factor is a correction for gas non-ideality, or deviations from the ideal-gas law. It is called the fugacity coefficient (φ). The second, the activity coefficient (γ), is a correction for interactions in the liquid phase between the different molecules.This modified or extended Raoult's law is then written:
Real solutions
Many pairs of liquids are present in which there is no uniformity of attractive forces i.e. the adhesive & cohesive forces of attraction are not uniform between the two liquids, so that they show deviation from the Raoult's law which is applied only to ideal solutions.





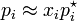

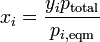





No comments:
Post a Comment