Statement:
At constant pressure, the volume of a given mass of an ideal gas increases or decreases by the same factor as its temperature on the absolute temperature scale.
Eg: Volume of Gas increases with increase in pressure.
which can be written as:
Graph Between Volume and Temperature:
Limitations:
- The ideal gas equation is usually derived from the kinetic theory of gases, which presumes that molecules occupy negligible volume, do not attract each other and undergo elastic collisions (no loss of kinetic energy); an imaginary gas with exactly these properties is termed an ideal gas. The behavior of a real gas is close to that of an ideal gas under most circumstances, which makes the ideal gas law useful.
- This law of volumes implies theoretically that as a temperature reaches absolute zero the gas will shrink down to zero volume. This is not physically correct, since in fact all gases turn into liquids at a low enough temperature, and Charles's law is not applicable at low temperatures for this reason.
- The fact that the gas will occupy a non-zero volume - even as the temperature approaches absolute zero - arises fundamentally from the uncertainty principle of quantum theory. However, as the temperature is reduced, gases turn into liquids long before the limits of the uncertainty principle come into play due to the attractive forces between molecules which are neglected by Charles's Law.

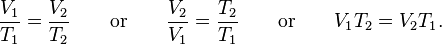




Can write in easy language
ReplyDelete